
Pipeline
EBI’s lead product successfully prevents tissue damage from fluid overload and promotes tissue repair. EBI’s product may find wide application in conditions that are the result of fluid overload and reduced hemodynamic stability, including respiratory viral infections with lung edema, such as seen with Covid-19. EBI’s lead target indication is acute kidney injury (AKI).

Acute Kidney Injury
The market for Acute Kidney Injury (AKI) therapeutics is large and steadily growing due to the rising incidence and recognition of the disease. Moreover, there is a need to prevent AKI in specific patient groups that are at high risk of developing AKI. One of these groups consists of patients undergoing (cardiac) surgery. As a result of increased diagnostics and awareness, the severity of the burden of AKI has become undeniable. In the past twenty years the number of AKI-associated hospitalizations and deaths has increased five-fold, up to around 700,000 deaths occurring annually in Europe, the US and Japan. Clearly, AKI has a striking socioeconomic and public health impact and is in desperate need of an effective treatment option.
Development Pipeline
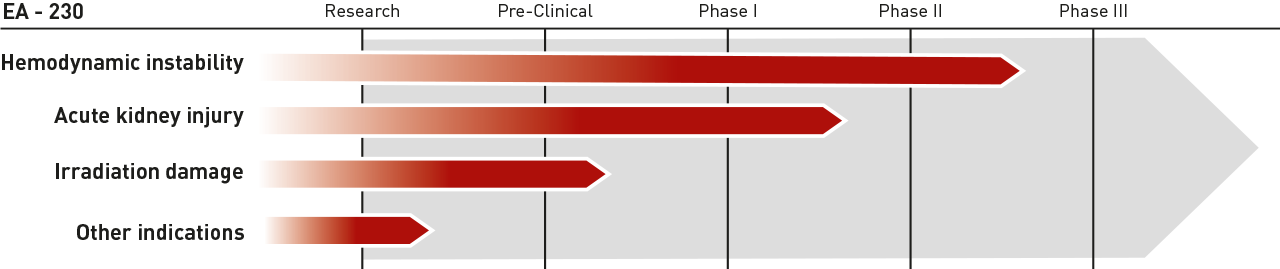
Irradiation damage
The second product application of EBI’s products is irradiation damage. Of the people diagnosed with cancer circa 50% require irradiation therapy, 60% of who are treated with curative intent. However, irradiation therapy is also related to severe risks. The response to radiotherapy is associated with several complications including debilitating damage to mucosal tissue. Currently, there are no safe and effective pharmacological agents to reduce irradiation toxicity that can be used in all patient groups.
Future indications
After successful development of EBI’s lead product for AKI and irradiation damage, EBI’s pipeline provides a path forward for the development of new and promising critical care therapies for multiple organ damage related to hemodynamic instability and fluid overload.

